Research
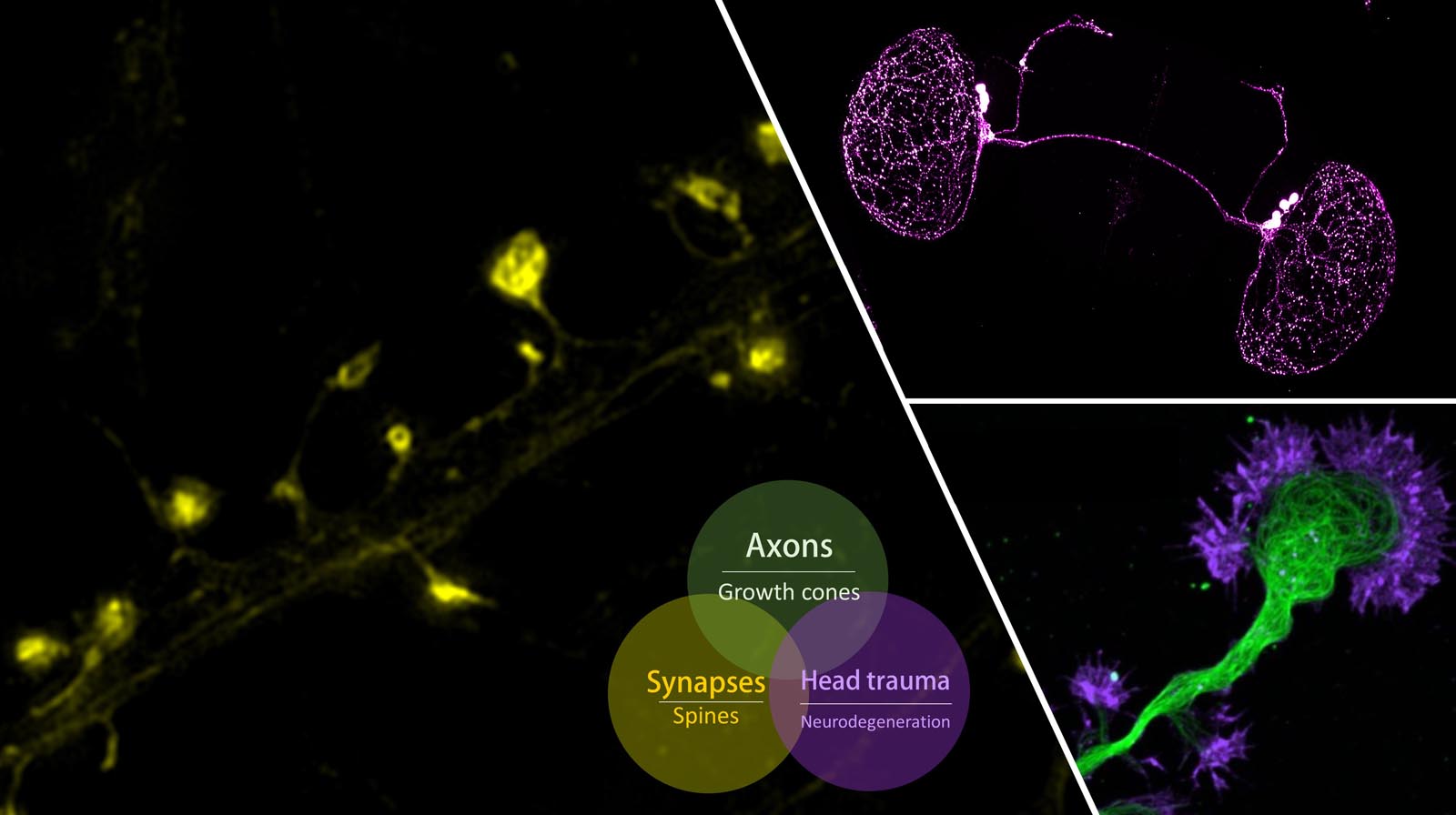
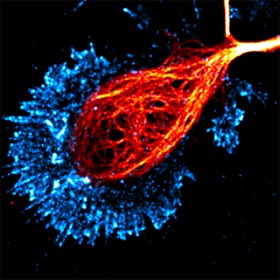 Over
a century ago, Ramon y Cajal made his landmark observations on the patterns of nerve process outgrowth and connectivity in developing brains and described the motile tip of
each elongating axon, the growth cone, as the responsible unit for axon elongation and pathfinding to the target cells. Developing axons are guided to their targets by a variety of
environmental cues, including long-range diffusible and short-range surface-bound molecules that can either attract or repel the axon. The presence of these guidance cues in temporal
and spatial patterns enables the growth cone to navigate through the complex environment of the developing embryo to reach its correct target. We investigate the signaling pathways and
cytoskeletal mechanisms that enable the growth cone to translate extracellular signals to directional movement during guidance.
Over
a century ago, Ramon y Cajal made his landmark observations on the patterns of nerve process outgrowth and connectivity in developing brains and described the motile tip of
each elongating axon, the growth cone, as the responsible unit for axon elongation and pathfinding to the target cells. Developing axons are guided to their targets by a variety of
environmental cues, including long-range diffusible and short-range surface-bound molecules that can either attract or repel the axon. The presence of these guidance cues in temporal
and spatial patterns enables the growth cone to navigate through the complex environment of the developing embryo to reach its correct target. We investigate the signaling pathways and
cytoskeletal mechanisms that enable the growth cone to translate extracellular signals to directional movement during guidance.
Featured publications
- Li X, Shim S, Hardin KR, Vanaja KG, Song H, Levchenko A, Ming GL, and Zheng JQ (2022). Signal Amplification in Growth Cone Gradient Sensing by a Double Negative Feedback Loop among PTEN,
PI(3,4,5)P3 and Actomyosin. Molecular and Cellular Neuroscience 123 (Dec).
- Guirland C, Suzuki S, Kojima M, Lu B, and Zheng JQ (2004). Lipid Rafts Mediate Chemotropic Guidance of Nerve Growth Cones. Neuron 42(1):51-62.
- Wen Z, Guirland C, Ming G-L, and Zheng JQ (2004). A CaMKII/Calcineurin Switch Controls the Direction of Ca2+-dependent Growth Cone Guidance. Neuron 43(6):835-846.
- Zheng JQ (2000). Turning of Nerve Growth Cones Induced by Localized Increases in Intracellular Calcium Ions. Nature 403:89-93 (Jan. 6).
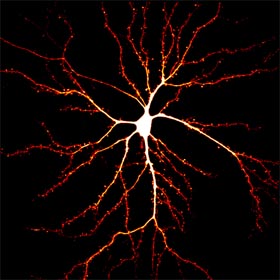 Synapses
represent the basic unit of neuronal communications and most of the excitatory synapses reside on dendritic spines, a type of dendritic protrusions that host neurotransmitter
receptors and other postsynaptic specializations. Synapses are plastic and undergo short- and long-term modifications during developmental refinement of neuronal circuitry, as well as during
learning and memory. Synaptic modifications involve both pre- and post-synaptic changes. Postsynaptically, modifications of the surface neurotransmitter receptors (numbers and properties) are
believed to be a key event underlying the changes in synaptic strength. In addition, dendritic spines undergo rapid changes in shape and size in association with synaptic modifications.
Our lab is interested in the cytoskeletal mechanisms that underlie the spine development during synaptogenesis and postsynaptic modifications during synaptic plasticity.
In particular, we have been studying the role of microtubules and the actin dynamics in spine formation, dynamics, and synaptic receptor trafficking.
Since many neurological disorders have been associated with alterations in synaptic connections, we hope that our studies will also shed light on brain development and functions under both
physiological and pathological conditions.
Synapses
represent the basic unit of neuronal communications and most of the excitatory synapses reside on dendritic spines, a type of dendritic protrusions that host neurotransmitter
receptors and other postsynaptic specializations. Synapses are plastic and undergo short- and long-term modifications during developmental refinement of neuronal circuitry, as well as during
learning and memory. Synaptic modifications involve both pre- and post-synaptic changes. Postsynaptically, modifications of the surface neurotransmitter receptors (numbers and properties) are
believed to be a key event underlying the changes in synaptic strength. In addition, dendritic spines undergo rapid changes in shape and size in association with synaptic modifications.
Our lab is interested in the cytoskeletal mechanisms that underlie the spine development during synaptogenesis and postsynaptic modifications during synaptic plasticity.
In particular, we have been studying the role of microtubules and the actin dynamics in spine formation, dynamics, and synaptic receptor trafficking.
Since many neurological disorders have been associated with alterations in synaptic connections, we hope that our studies will also shed light on brain development and functions under both
physiological and pathological conditions.
Featured publications
- Myers KR, Fan Y, McConnell P, Cooper JA, Zheng JQ (2022). Actin capping protein regulates postsynaptic spine development through CPI-motif interactions. Front Mol Neurosci. 2022 Sep 29;15:1020949.
- Omotade OF, Rui Y, Lei W, Yu K, Hartzell HC, Fowler VM, Zheng JQ (2018). Tropomodulin Isoform-Specific Regulation of Dendrite Development and Synapse Formation. J Neurosci. 2018 Nov 28;38(48):10271-10285.
- Lei W, Myers KR, Rui Y, Hladyshau S, Tsygankov D, Zheng JQ (2017). Phosphoinositide-dependent enrichment of actin monomers in dendritic spines regulates synapse development and plasticity.
J Cell Biol. 2017 Aug 7;216(8):2551-2564.
- Fan Y, Tang X, Vitriol E, Chen G, and Zheng JQ (2011). Actin Capping Protein is Required for Dendritic Spine Development and Synapse Formation. Journal of Neuroscience 31(28):10228-10233.
- Gu J, Lee CW, Fan Y, Komlos D, Tang X, Sun C, Yu K, Hartzell HC, Chen G, Bamburg JR, and Zheng JQ (2010). ADF/Cofilin-Mediated Actin Dynamics Regulate AMPA Receptor Trafficking during
Synaptic Plasticity. Nature Neuroscience 13(10):1208-1215.
- Lee CH, Han J, Bamburg, JR, Han L, Lynn R, and Zheng JQ (2009): Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin-Directed Vesicular Trafficking. Nature Neuroscience 12, 848 - 856.
- Gu J, Firestein BL, and Zheng JQ (2008): Microtubules in Dendritic Spine Development. Journal of Neuroscience 28: 12120-12124.
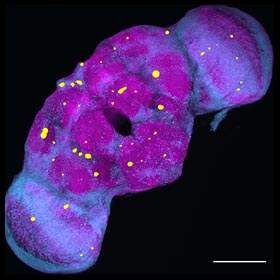 The progressive
loss of the function and number of neurons in the central nervous system is a hallmark event of a wide variety of neurodegenerative diseases leading to dementia, including Alzheimer’s disease.
Neurodegenerative disorders affect millions of people worldwide and the number is expected to increase substantially with the growth of the world population and increased life expectancy.
Major efforts have been directed to understand the etiology of various neurodegenerative conditions, but the challenge remains to elucidate the molecular and genetic mechanisms underlying the onset and progression
of neurodegeneration. Recent studies have identified many genetic mutations and risk factors associated with dementia and neurodegeneration, but they only account for a small percentage of overall cases.
It is believed that a variety of environmental factors, including chemical and physical insults, can trigger aberrant processes that may be latent at first but surface in late-life to disrupt various aspects
of neuronal structure and function, leading to neurodegenerative conditions. Physical blows to the head are known to cause immediate brain injury, as well as to lead to insidious long-term dementia, degeneration,
and disability. Even seemingly innocuous impacts to the head, while not causing immediate brain damage, could result in brain dysfunction and degeneration later in life. It is thus crucial to understand
the “hidden” processes triggered by these mild physical (or environmental) insults that can give rise to late-life neurodegenerative conditions.
The progressive
loss of the function and number of neurons in the central nervous system is a hallmark event of a wide variety of neurodegenerative diseases leading to dementia, including Alzheimer’s disease.
Neurodegenerative disorders affect millions of people worldwide and the number is expected to increase substantially with the growth of the world population and increased life expectancy.
Major efforts have been directed to understand the etiology of various neurodegenerative conditions, but the challenge remains to elucidate the molecular and genetic mechanisms underlying the onset and progression
of neurodegeneration. Recent studies have identified many genetic mutations and risk factors associated with dementia and neurodegeneration, but they only account for a small percentage of overall cases.
It is believed that a variety of environmental factors, including chemical and physical insults, can trigger aberrant processes that may be latent at first but surface in late-life to disrupt various aspects
of neuronal structure and function, leading to neurodegenerative conditions. Physical blows to the head are known to cause immediate brain injury, as well as to lead to insidious long-term dementia, degeneration,
and disability. Even seemingly innocuous impacts to the head, while not causing immediate brain damage, could result in brain dysfunction and degeneration later in life. It is thus crucial to understand
the “hidden” processes triggered by these mild physical (or environmental) insults that can give rise to late-life neurodegenerative conditions.
In the past, we have investigated the impairment of axonal trafficking by disease-associated molecules. We recently developed a novel mild head trauma model in Drosophila melanogaster that
enables us to investigate the late-life emergence of neurodegenerative conditions. Importantly, our data indicate the existence of sex differences in the development of neurodegenerative conditions after
early life exposure to mild physical impacts to the head. We are using this fruit fly model to further investigate the sex differences, aging effects, and the molecular and cellular mechanisms underlying the late-life emergence of neurodegeneration using a combination of molecular, genetic, and imaging approaches.
Featured publications
- Behnke JA, Ye C, and Zheng JQ (2021): Repetitive Mild Head Trauma Induces Activity Mediated Lifelong Brain Deficits in a Novel Drosophila Model. Scientific Reports. DOI: 10.1038/s41598-021-89121-7.
- Rui Y, Gu J, Yu K, Hartzell HC and Zheng JQ (2010). Inhibition of AMPA Receptor Trafficking at Hippocampal Synapses by beta-amyloid Oligomers: the Mitochondrial Contribution. Molecular Brain 3:10.
- Rui Y, Tiwari P, Xie ZP, and Zheng JQ (2006): Acute Impairment of Mitochondrial Trafficking by beta-amyloid Peptides in Hippocampal Neurons. Journal of Neuroscience 26(41): 10480-10487.

 Over
a century ago, Ramon y Cajal made his landmark observations on the patterns of nerve process outgrowth and connectivity in developing brains and described the motile tip of
each elongating axon, the growth cone, as the responsible unit for axon elongation and pathfinding to the target cells. Developing axons are guided to their targets by a variety of
environmental cues, including long-range diffusible and short-range surface-bound molecules that can either attract or repel the axon. The presence of these guidance cues in temporal
and spatial patterns enables the growth cone to navigate through the complex environment of the developing embryo to reach its correct target. We investigate the signaling pathways and
cytoskeletal mechanisms that enable the growth cone to translate extracellular signals to directional movement during guidance.
Over
a century ago, Ramon y Cajal made his landmark observations on the patterns of nerve process outgrowth and connectivity in developing brains and described the motile tip of
each elongating axon, the growth cone, as the responsible unit for axon elongation and pathfinding to the target cells. Developing axons are guided to their targets by a variety of
environmental cues, including long-range diffusible and short-range surface-bound molecules that can either attract or repel the axon. The presence of these guidance cues in temporal
and spatial patterns enables the growth cone to navigate through the complex environment of the developing embryo to reach its correct target. We investigate the signaling pathways and
cytoskeletal mechanisms that enable the growth cone to translate extracellular signals to directional movement during guidance.

