Contact
Francisco J. Alvarez, PhD
Professor and Vice Chair
Department of Cell Biology
Emory University School of Medicine
615 Michael Street
Room 605D
Atlanta, GA 30322-3110
Office:404-727-5139
Publications on:
My NCBI
Google Scholar
Research Interests
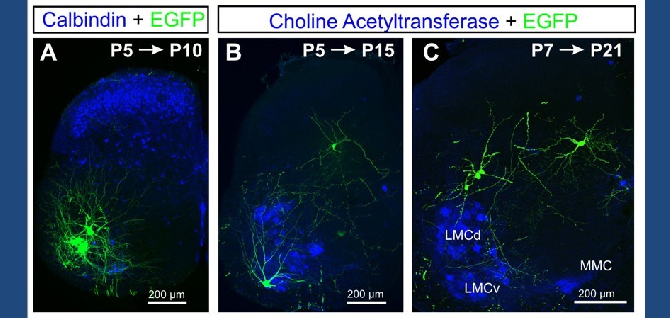
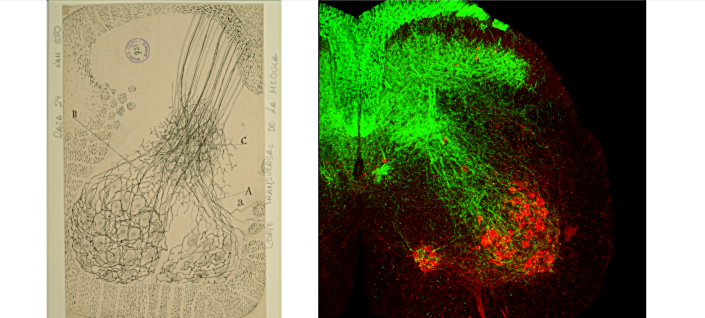
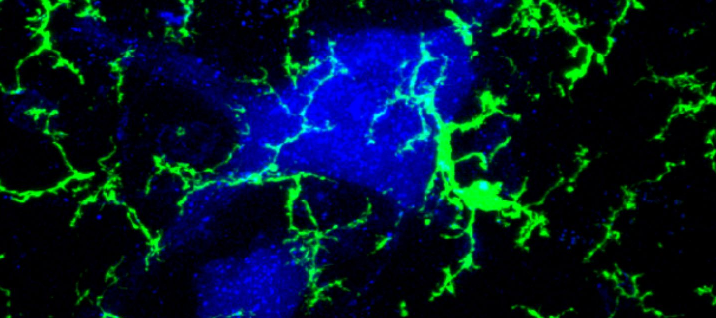
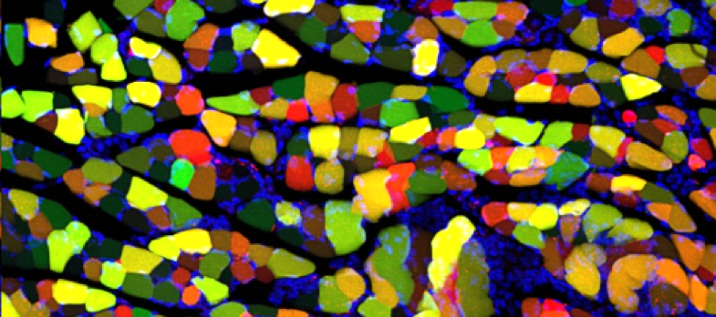
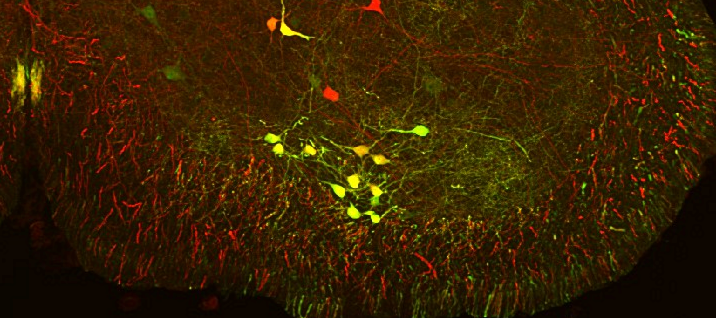
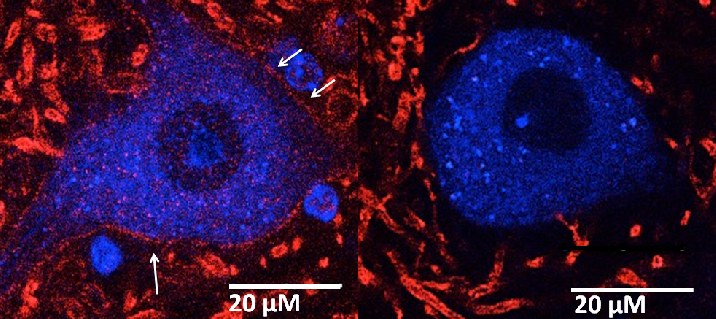
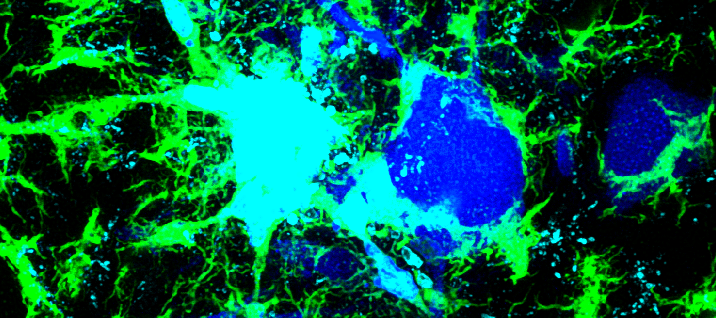
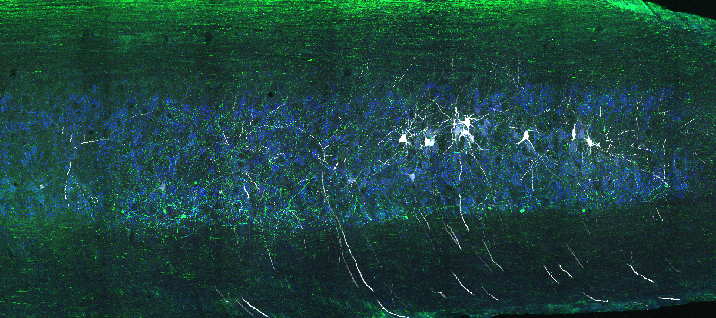
The Alvarez Lab studies the structure, development and function of spinal motor circuits. Spinal motoneurons innervate skeletal muscle and are the final common path by which our brain executes motor behaviors and interacts with the world around us. Coordinated movement depends on the pattern and timing of motor output from the spinal cord. Think about the many muscles that need to contract in a coordinated manner to place an index finger on the forehead. Motoneurons innervating different muscles need to fire in the appropriate sequence and frequencies to control both timing and strength of contractions in the different muscles moving a joint or a limb. This control is exerted by the neuronal networks of the spinal cord.
These networks are remarkable and allow us to maintain balance and posture, have volitional movements for either kicking a ball or playing the piano and also start rhythmic patterns like locomotion or scratching. Not surprisingly, they are extremely complex and although they have been investigated for over 100 years there is still much to learn about them. The lab is interested in the following questions:
Funding and Sponsors
Research Highlights and Lab News
CONGRATULATIONS TANA!!
On a great thesis and an excellent presentation. Good luck in all your future endeveours!
You can browse her work in the following preprints: