Targeting the PI3K signaling complex rescues FXS associated phenotypes in animal models
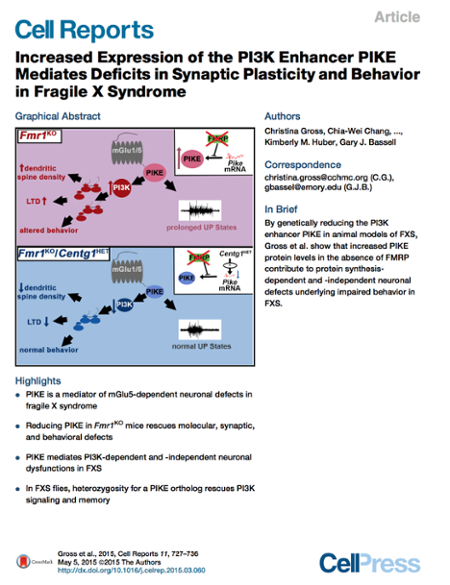
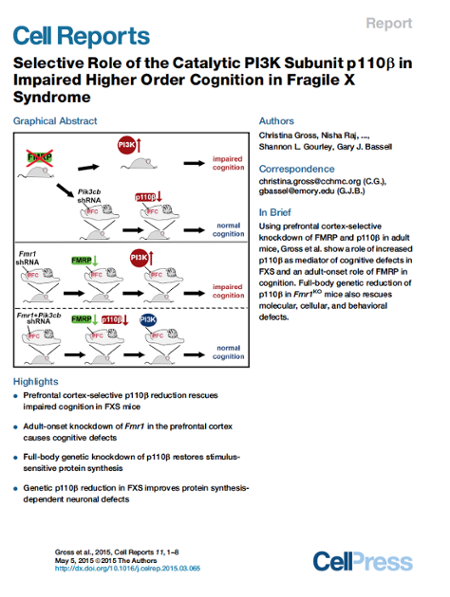
We hypothesize that targeting the downstream PI3K signaling complex that is associated with mGluRs may be an alternative therapeutic strategy to rescue phenotypes and treat FXS. In our first study on this project, we showed that FMRP controls the synthesis of the PI3K catalytic subunit, p110β, and that p110β-associated PI3K activity is upregulated at synapses in a mouse model of FXS. PI3K inhibitors ameliorated FXS-associated cellular and biochemical phenotypes (Gross et al., Journal of Neuroscience 2010). Subsequently we demonstrated that p110β is increased in human FXS patient lymphoblastoid cells and that subunit selective p110β inhibitors can rescue this excess protein synthesis. Subunit selective inhibitors were further shown to rescue impairments in synaptic protein synthesis in the mouse model of FXS (Gross et al., Molecular Medicine 2012). Recent work demonstrated that genetic reduction of p110β (Gross et al., Cell Reports 2015) or PIKE (Gross et al., Cell Reports 2015) can rescue FXS-associated phenotypes in animal models of FXS. These studies motivate the repurposing of p110β-selective antagonists for FXS, which are already being used in clinical trials with cancer patients. Future therapeutic strategies for FXS could include combinations of drugs targeting for example both PI3K signaling and mGlu5 receptors to ameliorate FXS-associated phenotypes including impairments in cognitive function.

Christina Gross, PhD